Scientists now on cusp of solving genetic diseases by snipping defective DNA
“It’s unbelievable,” Mr. Dupree, 26, said as he looked at his cells during a recent visit to a UT Southwestern gene-editing lab. “This could save so many lives one day.”
Advancements in gene editing, buoyed by the discovery of CRISPR technology that enables precise editing of the human genome, have put scientists on the cusp of solving some of mankind’s most devastating and baffling disorders. Among them is DMD, a muscle-withering disease that UT Southwestern geneticists have halted in animals and human cells through a single-cut gene-editing technique.
The next major step: a clinical trial that could change the prognosis for the most common fatal genetic disease in children and perhaps set the stage to treat other deadly diseases.
“Duchenne muscular dystrophy is a holy grail of genetic diseases, and we’re so close now to helping people like Ben,” said Dr. Eric Olson, Chair of Molecular Biology and an expert in genetic engineering at UT Southwestern. “Once we make headway with this condition, CRISPR in principle could be used for almost any disease in which we know the error in the DNA.”
Ben Dupree is introduced to Dr. Eric Olson, left, by Mr. Dupree’s cardiologist, Dr. Pradeep Mammen, right. Dr. Olson recalls, “From the first time I met ben, I felt we had a real connection.”
A rare mutation
Diagnosed at age 9, Mr. Dupree has spent most of his life dealing with the demoralizing effects of DMD. He recalls, even before diagnosis, the daunting task of keeping up with other children during soccer games or school exercise.
“Other kids would be running around, and I would be sitting with my hands on my knees trying to catch my breath,” he said. “It was hard to go through that.”
His family later learned this was far more serious than a lack of athleticism. Soon he was struggling to get up the stairs. Standing became a strenuous effort.
At the urging of an occupational therapist, Mr. Dupree’s mother sought neuromuscular testing and a biopsy that returned disturbing results: He had a rare genetic mutation that prevents his body from producing dystrophin, a protein that acts like a shock absorber for heart and muscle fibers when they expand and contract. Without dystrophin, the boy’s muscle fibers were falling apart – a condition that leads to muscle and heart failure and death by the early 30s, often much earlier.
Debbie Dupree did her best to hide the prognosis from her son as she began researching what could be done. She read about the latest findings on DMD, traveled to conferences, and met with doctors and other families dealing with the disease. Meanwhile, the boy’s muscles continued to deteriorate, and by middle school he was using a wheelchair full time.
“When a doctor tells you your child will most likely be gone within a decade because there is no treatment, you go into shock,” Mrs. Dupree said.
‘Patient Zero’
Mrs. Dupree, who had not heard of Duchenne before her son showed signs of the disease, was dismayed to learn of the lack of immediate options for DMD patients. Yet hope remained. Scientists had known for decades that DMD is caused by a defect in any of the 79 exons that comprise the dystrophin gene, and medical technology had recently reached a point where these defects could potentially be corrected through gene editing.
Coincidentally, one of the world’s leading experts on muscle disease lived in Mr. Dupree’s neighborhood and was researching this very strategy. Dr. Olson invited Mr. Dupree to UT Southwestern in 2015 to give a blood sample to test whether a new CRISPR method his lab developed could work on human cells.
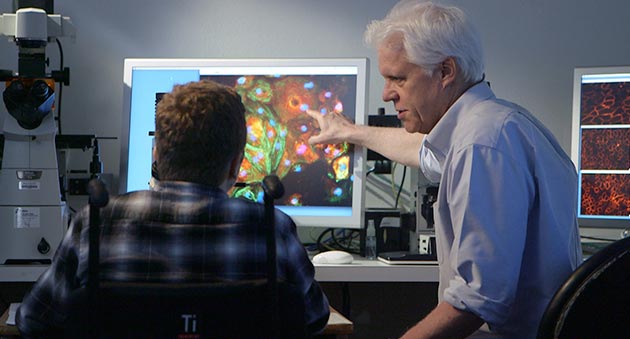
Dr. Olson’s team followed a series of biochemical steps to convert the sample into heart cells and used CRISPR to correct the exon defect and restore dystrophin. The team then invited Mr. Dupree to see the results through the microscope.
“That was one of the most inspiring moments of the work, to see Ben’s face when he saw his cells beating in a dish, producing the dystrophin that his body cannot make,” Dr. Olson said. “He was our Patient Zero. Now the challenge is to adapt the technique to safely deliver the gene-editing components into the human body.”
Dr. Olson’s work is a notable part of a series of programs at UT Southwestern aimed at developing genetic treatments for muscle diseases and a number of ultra-rare neurological conditions largely overlooked by the medical field.
UT Southwestern is trailblazing a series of clinical trials for the latter in which a single healthy gene missing from the patient’s DNA can be packaged into a harmless virus and delivered into cells. But scientists had to devise a different approach for DMD because the dystrophin gene – one of the largest in the human body – cannot fit inside the virus.
That was one of the most inspiring moments of the work, to see Ben’s face when he saw his cells beating in a dish, producing the dystrophin that his body cannot make.
Dr. Eric Olson
Dr. Olson’s team developed a CRISPR-Cas9 technique to fix the gene instead of adding a healthy copy into the cells. The technique requires two components: an enzyme called Cas9 that cuts DNA and a guide RNA that functions like a molecular GPS device to direct Cas9 to the specific exon to be edited.
Cas9 and the RNA are each loaded into an adeno-associated virus (AAV), with the RNA directing the Cas-9 to the mutated exon where it snips the DNA. The editing process bypasses the mutation and enables muscle fibers to produce dystrophin, with positive results documented in studies by Dr. Olson in dogs, mice, and human cells.
Success with these treatments could lay the foundation for the more intricate gene therapies of common conditions – from epilepsy to autism – involving multiple genes that are either mutated or missing. But for now Dr. Olson’s team is focused on saving patients like Mr. Dupree, one of about 300,000 people in the world with DMD.
“We’ve come a long way,” said Dr. Olson, whose lab has since edited cells taken from other patients with different exon mutations.
Mental strife
Mr. Dupree has come a long way as well. He overcame early mental strife to become a vocal advocate for DMD research and takes pride in helping younger patients deal with the complexities that lie ahead.
“I was able to come out of that dark place through the community of support,” Mr. Dupree said. “The people I came to know fostered a feeling that we’re all in this together and that brought me back to where I am today.”
He still keeps in touch with Dr. Olson, discussing the latest in science and sharing personal stories as well. It’s a relationship built on mutual respect and admiration, two people who are overcoming challenges to reach their ultimate goals.
“From the first time I met Ben, I felt we had a real connection,” Dr. Olson said. “It is probably surprising to people who realize many scientists will work their entire lives on a disease and not know someone personally who has that disease.”
Mr. Dupree continues to devote much of his time to helping others. He earned a bachelor’s degree in biochemistry – with the initial aim of researching DMD – and is now seeking a master’s in social work to counsel those with disabilities.
Regarding the prospects of overcoming DMD, Mr. Dupree takes nothing for granted.
“I’m not necessarily hoping for a cure, but for anything that could slow the progression of the disease,” he said. “Even if I’m not here to see the lifesaving treatment, my hope is that people being born now with the disease can still benefit.”



